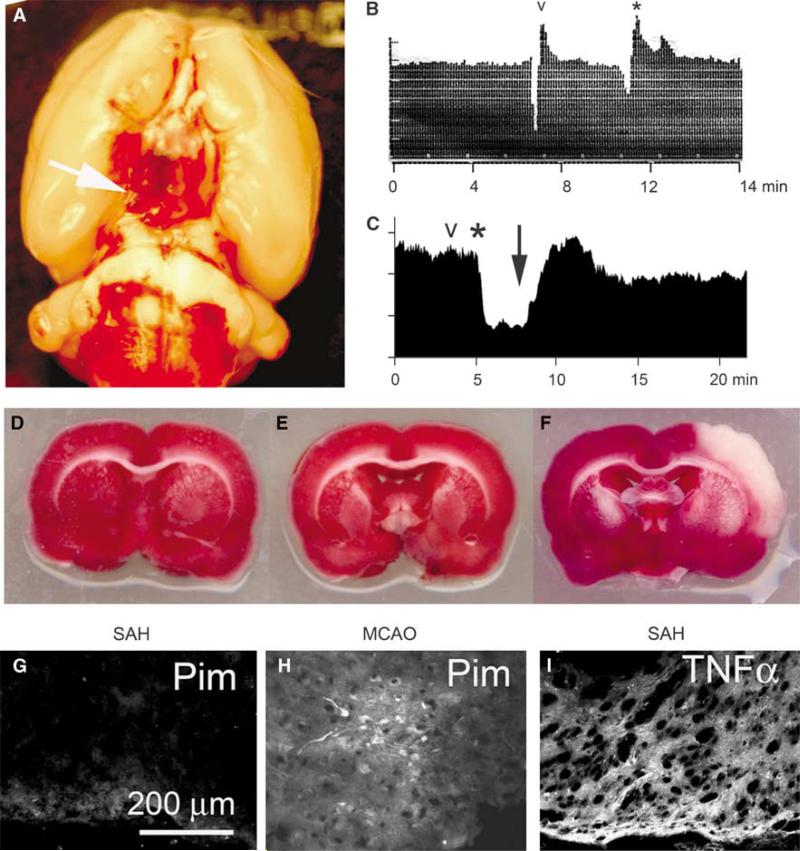
Subarachnoid hemorrhage (SAH) causes secondary mind damage resulting from vasospasm and irritation.
Here, we studied a rat mannequin of mild-to-moderate SAH supposed to attenuate ischemia/hypoxia to look at the function of sulfonylurea receptor 1 (SUR1) within the inflammatory response induced by SAH. mRNA for Abcc8, which encodes SUR1, and SUR1 protein have been abundantly upregulated in cortex adjoining to SAH, the place tumor-necrosis factor-alpha (TNFalpha) and nuclear issue (NF)kappaB signaling have been outstanding.
In vitro experiments confirmed that Abcc8 transcription is stimulated by TNFalpha. To examine the practical penalties of SUR1 expression after SAH, we studied the impact of the potent, selective SUR1 inhibitor, glibenclamide. We examined barrier permeability (immunoglobulin G, IgG extravasation), and its correlate, the localization of the tight junction protein, zona occludens 1 (ZO-1).
SAH induced a big enhance in barrier permeability and disrupted the conventional junctional localization of ZO-1, with glibenclamide considerably decreasing each results. In addition, SAH induced massive will increase in markers of irritation, together with TNFalpha and NFkappaB, and markers of cell damage or cell loss of life, together with IgG endocytosis and caspase-3 activation, with glibenclamide considerably decreasing these results.
We conclude that block of SUR1 by glibenclamide could ameliorate a number of pathologic results related to irritation that result in cortical dysfunction after SAH.
Contribution of vasogenic and mobile edema to traumatic mind swelling measured by diffusion-weighted imaging.
The contribution of mind edema to mind swelling in circumstances of traumatic mind damage stays a crucial drawback.
The authors consider that mobile edema, the results of complicated neurotoxic occasions, is the most important contributor to mind swelling and that vasogenic edema, secondary to blood-brain barrier compromise, could also be overemphasized.
The goal of this research, subsequently, was to quantify temporal water content material adjustments and doc the kind of edema that types in the course of the acute and late levels of edema growth following closed head damage (CHI).
The measurement of mind water content material was based mostly on magnetic resonance imaging-determined values of tissue longitudinal rest time (T1-weighted imaging) and their subsequent conversion to proportion of water, whereas the differentiation of edema formation (mobile vs. vasogenic) was based mostly on the measurement of the obvious diffusion coefficient (ADC) by diffusion-weighted imaging.
A brand new impact-acceleration mannequin was used to induce CHI. Thirty-six grownup Sprague-Dawley rats have been separated into two teams: Group I, management (six animals); and Group II, trauma (30 animals).
Fast ADC measurements (localized, single-voxel) have been obtained sequentially (each minute) as much as 1 hour postinjury. The T1-weighted photos, used for water content material willpower, and the diffusion-weighted photos (ADC measurement with typical diffusion-weighted imaging) have been obtained on the finish of the first hour postinjury and on Days 1, 3, 7, 14, 28, and 42 in animals from the trauma and management teams.
In the animals subjected to trauma, the authors discovered a major enhance in ADC (10 +/- 5%) and mind water content material (1.3 +/- 0.9%) in the course of the first 60 minutes postinjury. This is per a rise within the quantity of extracellular fluid and vasogenic edema formation because of blood-brain barrier compromise.
This transient enhance, nevertheless, was adopted by a seamless lower in ADC that started 40 to 60 minutes postinjury and reached a minimal worth on Days 7 to 14 (10 +/- 3% discount). Because the water content material of the mind continued to extend in the course of the first 24 hours postinjury (1.9 +/- 0.9%), it’s prompt that the decreased ADC indicated mobile edema formation, which began to develop quickly after damage and turned dominant between 1 and 2 weeks postinjury.
The research offers supportive proof that mobile edema is the most important contributor to posttraumatic swelling in diffuse CHI and defines the onset and period of the rise in mobile quantity.